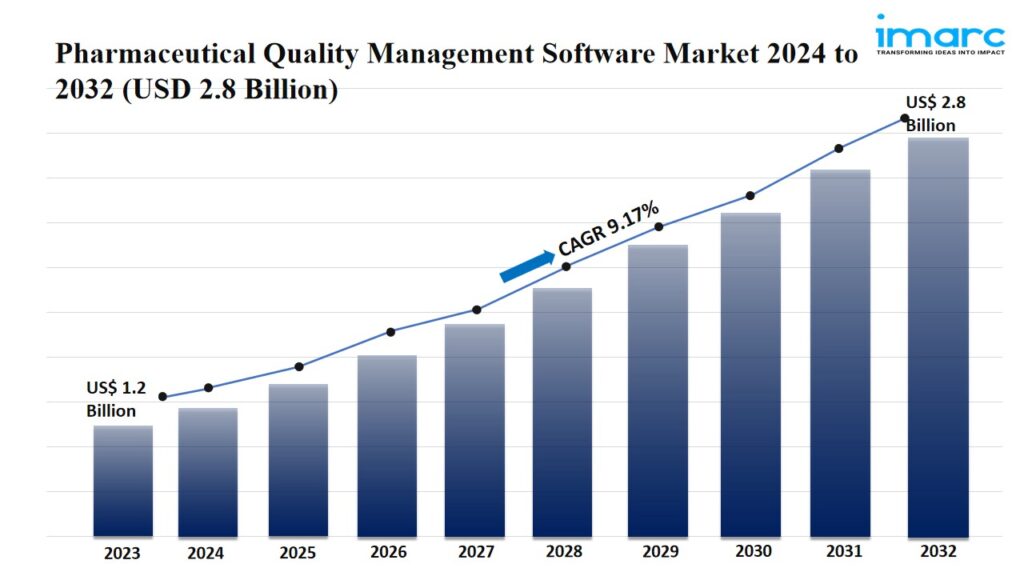
IMARC Group’s report titled “Pharmaceutical Quality Management Software Market Report by Solution Type (Corrective Action Preventive Action (CAPA) Management, Audit Management, Document Management, Change Management, Training Management, Risk Management, Complaints Management, Regulatory and Compliance Management, Non-Conformances Handling, Supplier Quality Management, Inspection Management, Equipment Management, and Others), Deployment Model (On-Cloud, Web-Based, On-Premises), Enterprise Size (Small and Medium-sized Enterprises, Large Enterprises), End User (Pharmaceutical, Life Sciences, and Biotechnology Companies, Contract Research Organizations, and Others), and Region 2024-2032”. The global pharmaceutical quality management software market size reached US$ 1.2 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 2.8 Billion by 2032, exhibiting a growth rate (CAGR) of 9.17% during 2024-2032.
For an in-depth analysis, you can refer sample copy of the report: https://www.imarcgroup.com/pharmaceutical-quality-management-software-market/requestsample
Factors Affecting the Growth of the Pharmaceutical Quality Management Software Industry:
- Rising Adoption of Cloud-based Solutions:
Cloud-based quality management software (QMS) solutions offer scalability to handle varying workloads and business needs without requiring significant infrastructure investments. Pharmaceutical companies can easily scale their operations up or down based on demand, making it cost-effective and efficient. Moreover, cloud solutions eliminate the need for upfront hardware costs and reduce information technology (IT) management overhead. Pharmaceutical companies can subscribe to a software-as-a-service (SaaS) model, paying for usage on a subscription basis, which often proves more cost-effective than maintaining on-premise systems.
- Technological Advancements:
Modern QMS platforms leverage advanced analytics tools to provide real-time insights into quality metrics and performance indicators. Pharmaceutical companies can generate customized reports, dashboards, and visualizations that help in monitoring quality trends, compliance status, and operational efficiencies. In line with this, QMS vendors are developing mobile applications that allow quality managers and personnel to access QMS functionalities on smartphones and tablets. Mobile apps facilitate on-the-go access to quality data, incident reporting, and task management, improving responsiveness and efficiency.
- Increasing Complexities in Supply Chain:
Pharmaceutical companies are outsourcing manufacturing and sourcing raw materials from worldwide suppliers. This globalized supply chain introduces complexities related to quality assurance, regulatory compliance, and supplier management. QMS software helps in standardizing quality processes across geographies and ensures consistency in product quality and compliance standards. In addition, with operations spread across multiple manufacturing sites, distribution centers, and contract manufacturing organizations (CMOs), ensuring consistent quality control becomes challenging. QMS software facilitates centralized quality management by standardizing processes, conducting remote audits, and maintaining visibility across the entire supply chain.
Leading Companies Operating in the Global Pharmaceutical Quality Management Software Industry:
- AssurX Inc.
- ComplianceQuest
- Dassault Systèmes SE (Dassault Group)
- ETQ LLC (Hexagon AB)
- Ideagen, IQVIA Inc.
- Mastercontrol Inc.
- Qualio
- Veeva Systems Inc
Pharmaceutical Quality Management Software Market Report Segmentation:
By Solution Type:
- Corrective Action Preventive Action (CAPA) Management
- Audit Management
- Document Management
- Change Management
- Training Management
- Risk Management
- Complaints Management
- Regulatory and Compliance Management
- Non-Conformances Handling
- Supplier Quality Management
- Inspection Management
- Equipment Management
- Others
Based on the solution type, the market has been divided into corrective action prevention action (CAPA) management, audit management, document management, change management, training management, risk management, complaints management, regulatory and compliance management, non-conformances handling, supplier quality management, inspection management, equipment management, and others.
By Deployment Model:
- On-Cloud
- Web-Based
- On-Premises
On the basis of the deployment model, the market has been classified into on-cloud, web-based, and on-premises.
By Enterprise Size:
- Small and Medium-sized Enterprises
- Large Enterprises
Based on the enterprise size, the market has been bifurcated into small and medium-sized enterprises and large enterprises.
By End User:
- Pharmaceutical, Life Sciences, and Biotechnology Companies
- Contract Research Organizations
- Others
Pharmaceutical, life sciences, and biotechnology companies represent the largest segment as these industries operate within stringent regulatory frameworks that demand rigorous quality control measures to ensure compliance with global standards, such as good manufacturing practices (GMP) and international organization for standardization (ISO) regulations.
Regional Insights:
- North America: (United States, Canada)
- Asia Pacific: (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe: (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America: (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position in the pharmaceutical quality management system market on account of a significant number of pharmaceutical companies ranging from large multinational corporations to small and mid-sized enterprises.
Global Pharmaceutical Quality Management Software Market Trends:
The growing integration of artificial intelligence (AI) and machine learning (ML) capabilities into QMS solutions automates and enhances quality management processes. AI-powered analytics can analyze vast amounts of quality data to identify trends, predict potential quality issues, and recommend preventive actions, thereby improving decision-making and proactive quality management.
Furthermore, Internet of Things (IoT) devices are used to monitor and collect real-time data from pharmaceutical manufacturing processes and equipment. QMS solutions integrated with IoT enable continuous monitoring of critical parameters, such as temperature, humidity, and pressure, ensuring adherence to quality standards and early detection of deviations.
Note: If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARCs information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the companys expertise.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
